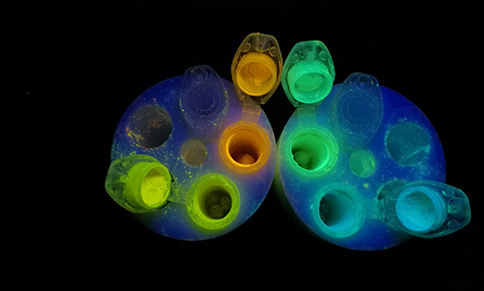
Luminescent compunds
Since the discovery of electroluminescent properties of tris(8-oxyquinolinato)aluminium (AlQ3) wide research have been conducted on application of metal chelate complexes in optoelectronics. Organoboron compounds show analogous Lewis acidic behaviour to heavier Group 13 congeners and they are able to form complexes with various chelating ligands. In addition, they turned out to be more thermally and chemically resistant compared to aluminium, gallium or zinc complexes. Due to these fundamental features, boron compounds seem to be ideal candidates for the design of fluorescent materials with target applications in optoelectronic devices including organic light emitting diodes (OLEDs), organic field-effect transistors as well as photoresponsive materials. Furthermore, macroscopic properties of organoboron complexes such as absorption/emission wavelength, quantum yield of emission and charge transport capabilities can be properly modified for the desired functionality by altering the structure of the compound. The most common strategy involves the diversification of the ligand structure. For instance, recently we have synthesized a group of strongly fluorescent (QY 60-80%) orange-to-red light emitting bis(boranils) derived from 1,5-dihydroxynaphthalene-2,6-dicarboxaldehyde. The promising features of obtained systems, i.e., improved stability (thermal, hydrolytic, electrochemical) and strong fluorescence in a red region of the visible spectrum, would be beneficial for a construction of highly efficient organic red-light emitting diodes. (Journal of Organic Chemistry, 2017, 82, 8234−8241 pub 5).
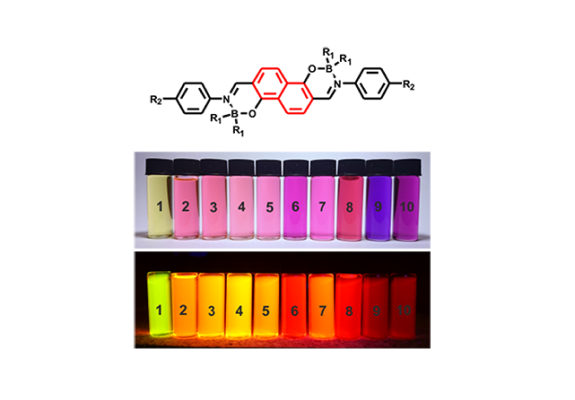
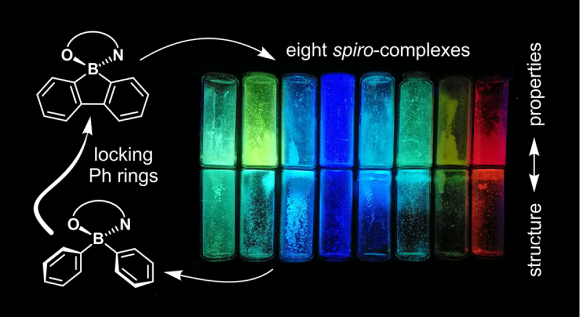
The second strategy is based on the modification of the organoboron structure. Against general conviction on weak structural-property dependence of various boron-functionalized derivatives, we have found that specific modification of organoboron structure, i.e. incorporation of a boron atom into a rigid 5- or 6-membered boracyclic ring, can significantly affect the physicochemical properties of a system. Encouraged by these results we have obtain series of boracyclic compounds including 9,10-diboraanthracenes (J. Mater. Chem. C, 2015, 3, 1354–1364 pub 8), 9-borafluorenes, 9,10-silaboraanthracene, and benzoxaborins, which were further combined with already in-use or developed by us ligands. We have found that obtained complexes are characterized by enhanced thermal stability and are strongly resistant to chemical degradations. The comparison to BPh22 systems shows that the absorption/emission profiles are generally preserved (5-15 nm blue- or red-shifts are observed), while fluorescence quantum yields strongly vary and depend on the ligand selection (both amplification and quenching effects are observed). It seems that organoboron and ligand moieties cannot be considered independently, and they have to be properly matched to amplify the complex properties. Selected systems were used for the construction of prototypical OLED devices. Furthermore, theoretical calculations suggest that they are promising candidates for third generation emitters featuring Thermally Activated Delayed Fluorescence (TADF) properties.
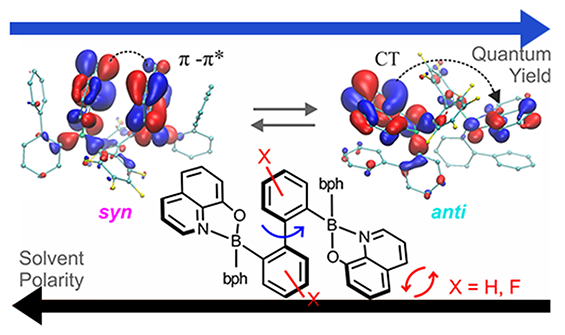
In our very recent studies we have realized that the conformation of the molecule can be another very important factor that can significantly influence the photophysical properties. Furthermore, it can be controlled by solvent polarity. (Dalton Trans., 2018, 47, 15670-15684, pub 1) These projects are supported by the National Science Centre (Narodowe Centrum Nauki, Grant No. UMO-2015/19/D/ST5/00735).
That's how it looks like
Antimicrobial agents
Currently, we are strongly interested in development of novel boraheterocycles as potential antimicrobial agents. This project is carried out with the strong participation by our collaborators Prof. Stefan Tyski and Dr Agnieszka E. Laudy from the Faculty of Pharmacy, Medical University of Warsaw. In 2015, We published the synthesis and biological activity of functionalized benzosiloxaboroles - the silicon analogues of benzoxaboroles. A detailed discussion of the mechanism of this reaction was published in our recent paper (Dalton Trans., 2018, 47, 3705−3716 pub 3, Eur. J. of Org. Chem., 2017, 818–826 pub 6, Eur. J. Med. Chem., 2019, 171, 11-24). These researches are supported by the National Science Centre (Narodowe Centrum Nauki, Grant No. UMO-2018/29/B/ST5/02108)
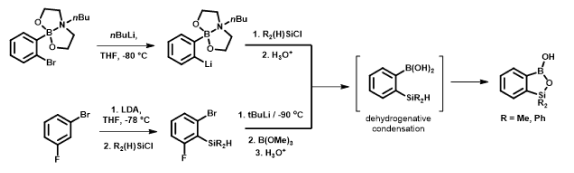
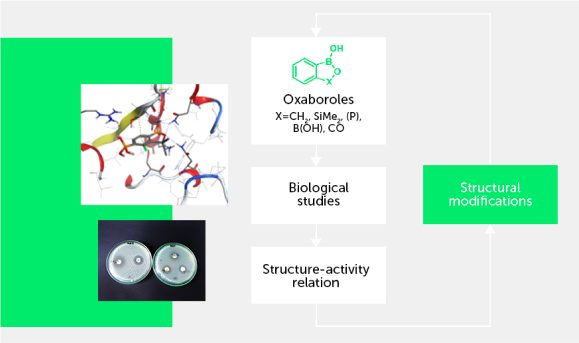
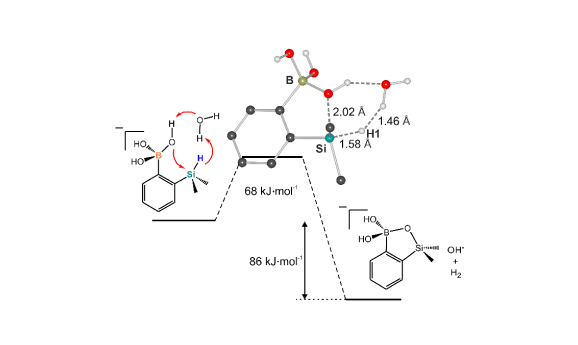
The developed protocols allowed us to obtain series of relatively simple halogenated benzosiloxaboroles as well as selected functionalized derivatives. The initial results show that obtained benzosiloxaboroles are promising antifungal and antibacterial agents. Despite a structural analogy to benzoxaboroles, target siloxaboroles are characterized by quite different physicochemical properties. In our initial results we have found that the presence of B-O-Si linkage results in the increased Lewis acidity with respect to benzoxaboroles. The strategy for the synthesis of these compounds is also different, which opens possibilities for new functionalized derivatives and their further applications. Selected compounds showed good antifungal activity and thus they can be regarded as potential small-molecule therapeutic agents. Our initial results indicate that low Minimal Inibitory Concentration (MIC) values of a few mg/L (or even less) can be achieved for selected halogenated benzosiloxaboroles. For instance, 5,6-difluoro derivative showed high activity against Candida tropicalis and Candida quilliermondii with MICs equal to 0.78 and 6.25 mg/L, respectively. These compounds were also demonstrated to be effective receptors for biologically relevant diols. For instance, the binding constants of 7-fluorobenzosiloxaborole with dopamine, ribose, and adenosine under neutral pH conditions were 13700, 4830 and 2030 M-1, respectively (Organometallics, 2015, 34, 2924–2932 pub 9). These values are high when compared to figures reported for many other organoboron receptors. It has been shown that the key access to the activation of benzoxaboroles toward antimicrobial properties lies in a proper substitution at the aromatic ring or carbon atom of borole ring. This enables fine tuning of biological activity and opens applicability of these compounds for the treatment of different diseases. In the case of benzosiloxaboroles we have found that their antimicrobial activity is quite different than that of benzoxaboroles. For instance, within the group of simply halogenated benzoxaboroles the most active ones bear substituent attached at 5 or 6 position of aromatic ring and fluorine substitution is more beneficial than chlorine (or other halogens). According to our preliminary results, it seems that the most active are benzosiloxaboroles bearing, besides some other functional groups, a halogen substituent (chlorine or fluorine) in the vicinity of a silicon atom (position 7). Furthermore, the biological selectivity of benzoxa- and siloxaboroles seems to be different, too. It means that the respective structural analogs are active against different microbial pathogens.
Microporous materials
We concentrate our research on the design of novel functional materials featuring porous structure capable of storage or separation of technically and environmentally important gases. Particularly, we are interested in Covalent Organic Frameworks and, very recently, hybrid systems incorporating metal atoms in their structures. We have studied hybrid boron-triazine COFs, combining advantages of both classes of materials – facile synthesis and exceptional thermal stability (ACS Appl. Mater. Interfaces, 2017, 9, 31129−31141 pub 4). Periodic theoretical calculations revealed that the eclipsed structures are more stable than staggered ones reflecting the effect of stacking interactions between adjacent rings. A closer inspection of possible stacking modes indicates that relatively weak but specific interactions can be utilized for controlling the interlayer organization of 2D COF materials.
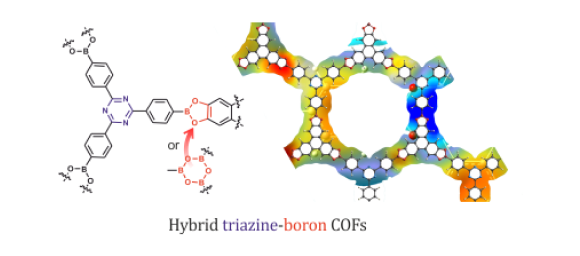
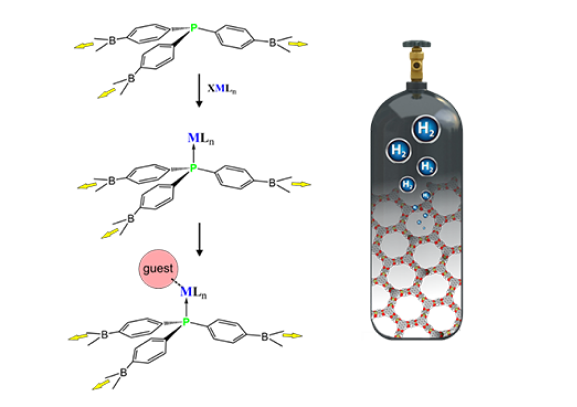
We concentrate our research on the design of novel functional materials featuring porous structure capable of storage or separation of technically and environmentally important gases. Particularly, we are interested in Covalent Organic Frameworks and, very recently, hybrid systems incorporating metal atoms in their structures. We have studied hybrid boron-triazine COFs, combining advantages of both classes of materials – facile synthesis and exceptional thermal stability (ACS Appl. Mater. Interfaces, 2017, 9, 31129−31141 pub 4). Periodic theoretical calculations revealed that the eclipsed structures are more stable than staggered ones reflecting the effect of stacking interactions between adjacent rings. A closer inspection of possible stacking modes indicates that relatively weak but specific interactions can be utilized for controlling the interlayer organization of 2D COF materials.
We concentrate our research on the design of novel functional materials featuring porous structure capable of storage or separation of technically and environmentally important gases. Particularly, we are interested in Covalent Organic Frameworks and, very recently, hybrid systems incorporating metal atoms in their structures. We have studied hybrid boron-triazine COFs, combining advantages of both classes of materials – facile synthesis and exceptional thermal stability (ACS Appl. Mater. Interfaces, 2017, 9, 31129−31141 pub 4). Periodic theoretical calculations revealed that the eclipsed structures are more stable than staggered ones reflecting the effect of stacking interactions between adjacent rings. A closer inspection of possible stacking modes indicates that relatively weak but specific interactions can be utilized for controlling the interlayer organization of 2D COF materials.
Crystal engineering
Crystal engineering aspires to understand the influence of non-covalent interactions on the macroscopic properties of organic crystals (density, solubility, thermal and mechanical behaviour, electronic and optical properties), which would help to design materials possessing desired parameters. In our study we focus particular attention on structure-energy-property dependence in the solid-state structures of organoboron compounds and related systems. We have studied the effect of the fluorine substitution and the role of the water molecules on the crystal organization in the series of 1,4-phenylenediboronic acids (Cryst. Growth Des., 2012, 12, 3720–3724 pub 23). In another study we have found that 1,2-phenylenediboronic acid crystallizes in 3 different solvatomorphic forms (Cryst. Growth Des., 2013, 13, 4181–4185. Pub 19). One of them constitutes very interesting nanotubular hydrogen-bonded organic framework (HOF). In contrast to carbon nanotubes, the intermolecular connections are based strictly on hydrogen-bonding interactions. Furthermore, experimental and computational analyses showed that such nanotubes may host water clusters similar to the ones found in the hexagonal structures of ice. Further studies on the fluorinated 1,2-phenylenediboronic acid revealed that the boron Lewis acidity significantly influences the structural behaviour of these compounds (Organometallics, 2014, 33, 1608–1616 Pub 15). This is dictated by the superposition of two major factors: the vicinity of two boronic groups and the strong effect of fluorine substituents. Solution NMR studies indicate that these compounds equilibrate with the corresponding cyclic semi-anhydrides possessing the benzoxadiborole structure. Ab initio calculations showed that the stability of semi-anhydrides is improved by fluorination of the aromatic ring and complexation of one of the boron centres with water. This was demonstrated by crystal structure determination of tetrafluoro-1,2-phenylenediboronic acid. The coordinated water molecule participates in very strong intermolecular hydrogen-bonding with the OH group bonded to the 4-coordinate boron center. This indicates that in fact this compound is an oxonium, i.e., Brønsted acid, which is exceptional for boronic acids. Under different crystallization conditions, tetrafluoro-1,2-phenylenediboronic acid dimerizes by aggregation of boronic groups, which leads to the formation of an uncommon 8-membered B4O4 ring. Boron Lewis acidity may also strongly influence the behaviour of boracyclic compounds. Our results demonstrate that pyridoxaboroles can serve as self-complementary tectons for generation of molecular networks through N–B coordination and/or hydrogen bonds (Dalton Trans., 2015, 44, 16534-16546 Pub 10). In a different work we have studied a competition between hydrogen (O–H…O, O–H…F) and halogen (Cl…Cl, O…Br) bonding interactions that influences the molecular arrangement in the crystal structures of a series of boranthrenes (Acta Crystallogr., 2014, B70, 157–171 pub 14). In turn, detailed investigations of crystal structure of terakis(thienylsilanes) using a combination of experimental and computational procedures revealed the interesting structure-property relationship (Cryst. Growth Des., 2016, 16, 4292−4308 Pub 7). Despite the fact that studied systems show a very high level of structural similarity, they revealed distinct physicochemical behaviour (different melting points and enthalpies of fusion, temperature-dependence unit-cell evolution, solubility). This is due to a different distribution of C−H···C(π), S···C(π), and S···S interactions within the corresponding crystal structures.
Synthesis & mechanistical studies
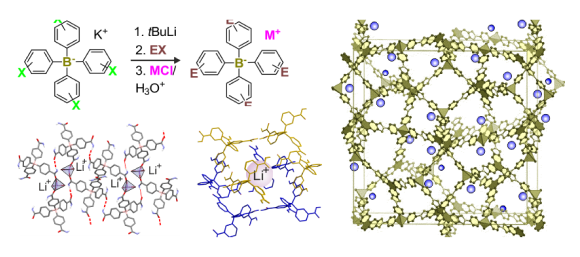
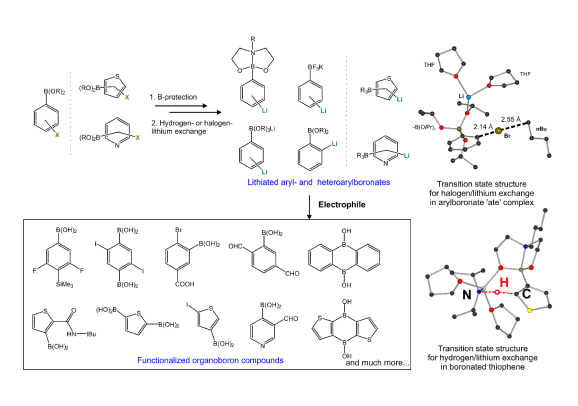
We are strongly involved in elaboration of regioselective protocols for generation of bimetallic lithium-boron compounds as versatile reagents for the preparation of functionalized organoboron and organosilicon systems. Since a three-coordinate boron atom is susceptible to nucleophilic attack, it requires protection which can be accomplished by conversion to derivatives bearing coordinatively saturated four-coordinate boron atom such as organo(trifluoro)borates (RBF3K), aryl(trialkoxy)borates (ArB(OR)3Li; so-called „ate”-complexes) or boronic esters with aminoalcohols featuring intramolecular N-B coordination (e.g., ArB(OCH2CH2NBu). We used for the first time the metalation reaction for the generation of aromatic lithium-boron reagents - lithiated haloarylboronic esters with N-butylodietanoloamine (Eur. J. Org. Chem. 2009, 4325) and found that deprotonation was effective only for dihalogenated substrates due to a sufficient CH-acidity of the aromatic ring. In addition, we found that the formation of bimetallic systems of the type Li[(LiAr)B(OR)3] was effective using n-BuLi and diiodobenzenes or activated dibromobenzenes (Eur. J. Org. Chem. 2008, 3171). It should be stressed that the developed one-pot protocol can be interesting from the technological perspective as it enables the preparation of various functionalized arylboronic acids from readily accessible and relatively cheap substrates without the need for isolation and purification of intermediates. Later on, we obtained analogous „ate”-complexes Li[(LiAr)PhB(OR)2] and their synthetic utility was confirmed by conversion to a series of unsymmetrically substituted diaryloborinic derivatives. Such compounds are susceptible to oxidative or hydrolytic degradation, and therefore they were finally isolated in the form of relatively stable esters with 8-hydroxyquinoline (RAr)PhB(8-ox), R = SMe, SiMe3, CHO, CONHt-Bu (J. Organomet. Chem. 2012, 711, 1). During these studies we raised a problem of potential directing effects of boron-based functional groups in deprotonation reactions. In the case of thiophene derivatives bearing B(OR)3Li and B(OCH2CH2)NBu moieties at the 3-position we observed metalation at the 2-position (Eur. J. Org. Chem. 2012, 2208 pub 22). Theoretical DFT calculations revealed that these groups decrease CH-acidic properties of thiophene ring and destabilize (to a varying extent) respective thienyl carbanions due to a positive inductive effect. On the other hand, the formation of an intramolecular dative (B)O…Li bonds leads to a significant stabilization of these derivatives in which the lithium atom is located in the vicinity of a boronate group. In a subsequent work (Eur. J. Org. Chem. 2013, 3023 pub 20), we showed that boron-based groups also had a strong impact on the regioselectivity of bromine-lithium exchange. Thus, for a series of 1,4-dibromobenzenes substituted at the 2-position with B(OR)3Li, B(OCH2CH2)NBu and BF3K groups, we observed deactivation of bromine-lithium exchange ortho to the former one. In contrast, the exchange occured regioselectively ortho with respect to the BF3K group in accordance with results of DFT calculations of the reaction kinetics showing also stabilization of the respective aryllithium product through an intramolecular (B)F…Li coordination. Extension of these studies resulted in development of a new convenient method for the synthesis of functionalized 9,10-dihydroxy-9,10-diboraanthracenes. It involves the treatment of 2-halophenylboronic esters (Hal = Br or I) with t-BuLi in THF at low temperature (Eur. J. Org. Chem. 2013, 8315 pub 21). In the initial stage of the reaction pathway ortho-lithiated phenylboronic esters are formed. These reactive intermediates feature three-coordinate Lewis acidic boron in the vicinity of strongly nucleophilic lithiated carbon atom. Overall, the entire reaction sequence can be regarded as the tandem process eventually leading to ring closure and formation of diboraanthracene scaffold.
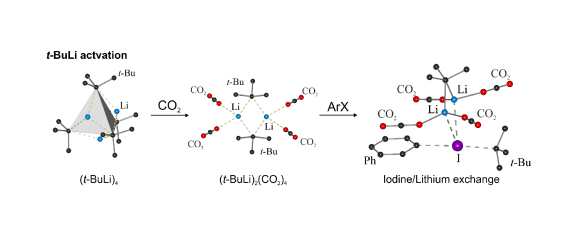
In addition, our continued interest in polylithiated aromatics has prompted us to investigate halogen-lithium exchange as a potential method for the synthesis of 1,2-dilithiobenzene (Eur. J. Org. Chem. 2014, 4562). Reactions of haloarenes with t-BuLi in pentane are kinetically supressed at low temperatures (below -60 °C) even in the case of the use of very reactive substrates such as 1,2-diiodobenzene and bromopentafluorobenzene. However, we observed that halogen-lithium exchange occured effectively after treatment of haloarene/t-BuLi mixtures with a large excess of CO2. This was evidenced by final isolation of respective aromatic carboxylic acids, e.g., phthalic acid was obtained from 1,2-diiodobenzene. The use of CO2 as an electrophilic trap in reactions with organolithium and magnesium compounds is of course one of classic approaches to carboxylic acids. An intriguing novel fact is that CO2 can act as an activator of halogen-lithium exchange despite its strongly electrophilic character. The observed effect is rather weak and can be observed only for activated haloarenes which is in accordance with the results of DFT calculations. This implies its limited importance with respect to practical applications. However, it deserves attention as it sheds new light on the mechanism of halogen-lithium exchange reactions, especially in the context of interactions of aggregated alkyllithiums with electrophilic reagents simultaneously featuring weak donor properties.
In addition, our continued interest in polylithiated aromatics has prompted us to investigate halogen-lithium exchange as a potential method for the synthesis of 1,2-dilithiobenzene (Eur. J. Org. Chem. 2014, 4562). Reactions of haloarenes with t-BuLi in pentane are kinetically supressed at low temperatures (below -60 °C) even in the case of the use of very reactive substrates such as 1,2-diiodobenzene and bromopentafluorobenzene. However, we observed that halogen-lithium exchange occured effectively after treatment of haloarene/t-BuLi mixtures with a large excess of CO2. This was evidenced by final isolation of respective aromatic carboxylic acids, e.g., phthalic acid was obtained from 1,2-diiodobenzene. The use of CO2 as an electrophilic trap in reactions with organolithium and magnesium compounds is of course one of classic approaches to carboxylic acids. An intriguing novel fact is that CO2 can act as an activator of halogen-lithium exchange despite its strongly electrophilic character. The observed effect is rather weak and can be observed only for activated haloarenes which is in accordance with the results of DFT calculations. This implies its limited importance with respect to practical applications. However, it deserves attention as it sheds new light on the mechanism of halogen-lithium exchange reactions, especially in the context of interactions of aggregated alkyllithiums with electrophilic reagents simultaneously featuring weak donor properties.
Optimisation & scale-up chemical reactions
Group members gained a strong experience in development of large scale procedures for the synthesis of fine chemicals. This work was conducted in the period 1992-2017 in the framework of a long term collaboration with SIGMA-ALDRICH chemical company (U.S.A, since 2015 a part of Merck Co.) and coordinated by the former group leader Prof. J. Serwatowski. We elaborated numerous protocols for the multigram (up to 1,500 g) preparation of organoboron compounds such as arylboronic acids and their derivatives (e.g., pinacol and MIDA esters). Other types of chemicals included organotins and various heteroaromatic compounds based on pyridine, quinoline, pyrimidine, pyrazine, pyrrole, indole, furan, and thiophene cores. Obtained compounds were added to the list of products being a part of commercial offer of SIGMA-ALDRICH.
Contact
Faculty of Chemistry
Warsaw University of Technology
Department of Physical Chemistry
Noakowskiego 3
00-664 Warsaw, Poland
Prof. Sergiusz Luliński
phone: +48 22 234 7273